I am a PhD Candidate at
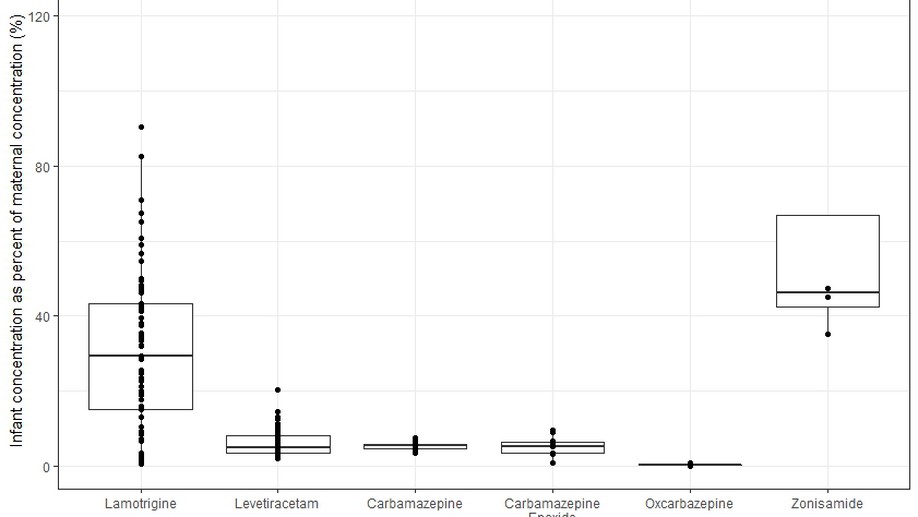
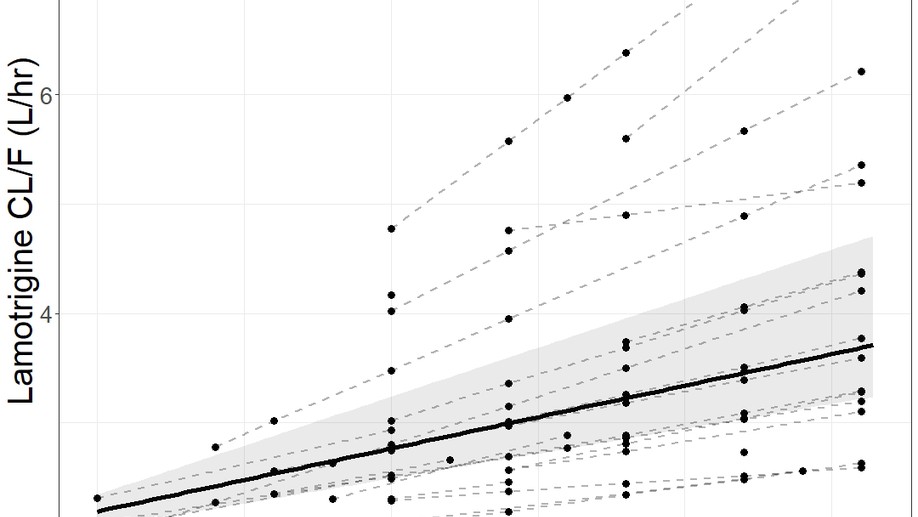
Angela Birnbaum Lab, University of Minnesota. My research interests include modeling and simulation, pharmacometrics and quantitative clincal pharmacology. My graduate research is focused on using model based methods to characterize pharmacokinetic and pharmacodynamic changes of antiseizure medicines in special populations of pregnancy, post-partum and breastfeeding infants.
I also serve as the current Chair of
ISoP Student Community.
Interests
- Modeling and Simulation
- Model Infromed Drug Development
- Quantitative Clinical Pharmacology
Education
PhD Experimental and Clinical Pharmacology (Minor in Biostatistics), 2016-2020
University of Minnesota
Doctor of Pharmacy (PharmD), 2016
Manipal University, India